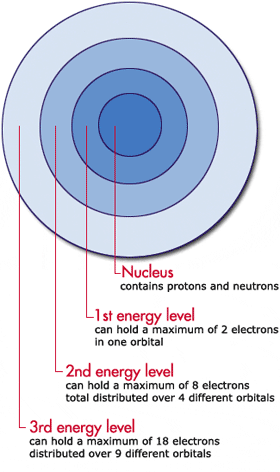
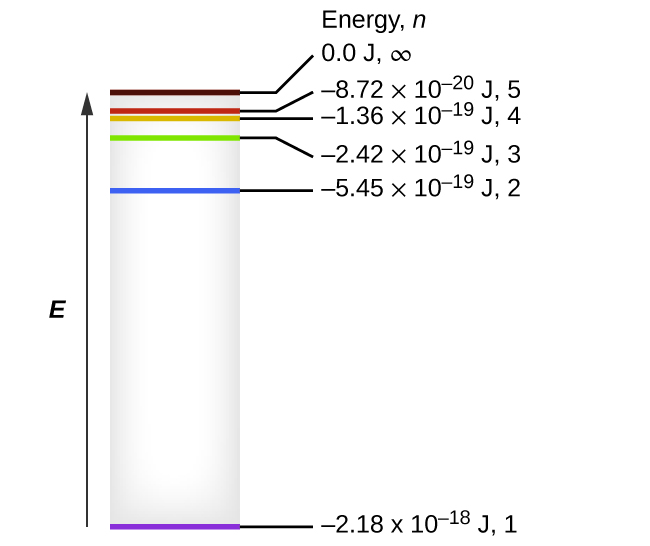
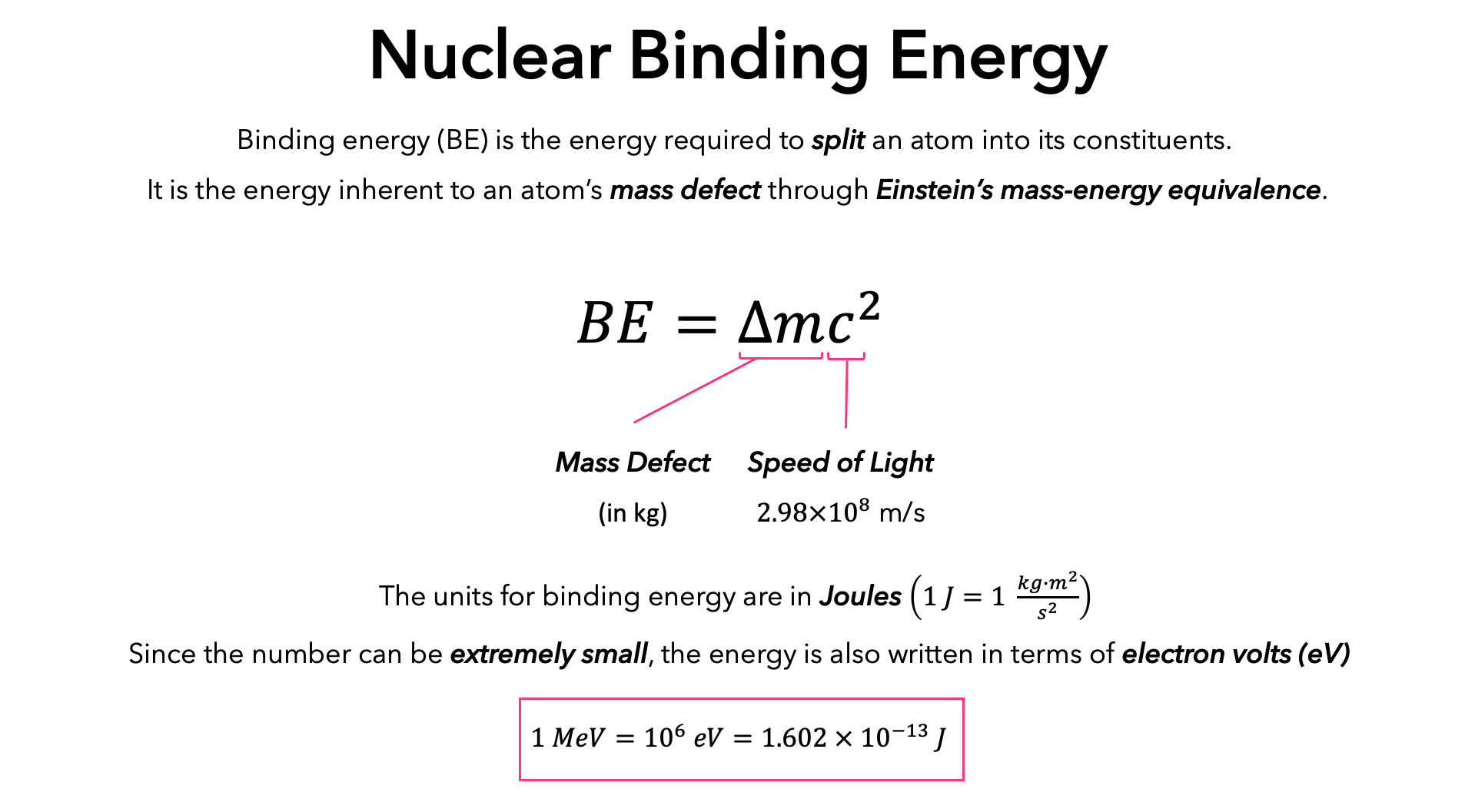
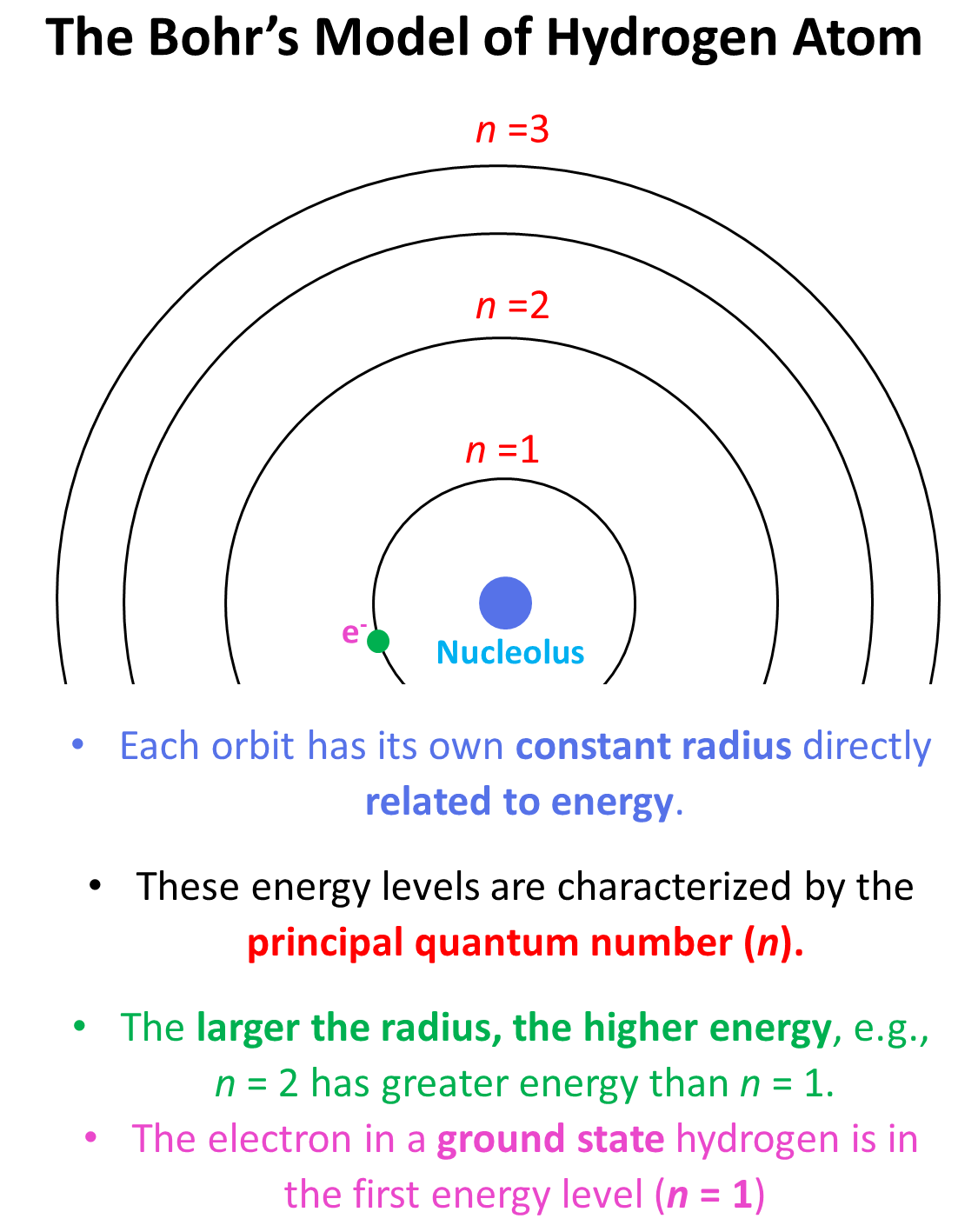
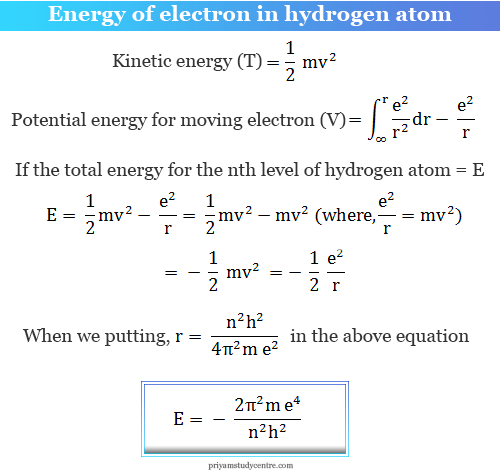
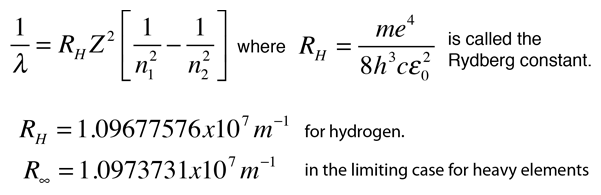SOLVED: Atomic Spectrum Report Sheet Name RonelQuebRal Calculate the ten lowest energy levels for the hydrogen atom. Start with n = 1 and carry the appropriate number of significant figures. Enter your

Calculate the energy required to excite an electron in hydrogen atom from the ground state to - YouTube

Question Video: Determining the Relationship between the Atomic Radius and the Ionization Energy | Nagwa
Using Bohr's formula for energy quantization, the ionisation potential of first excited state of hydrogen atom is: . (1) 13.6V, (2) 3.4V, (3) 2.6V, (4) 1.51V

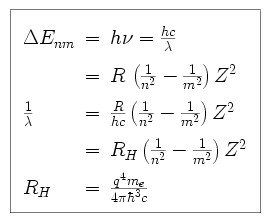
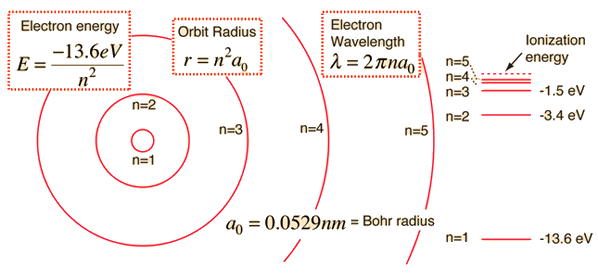

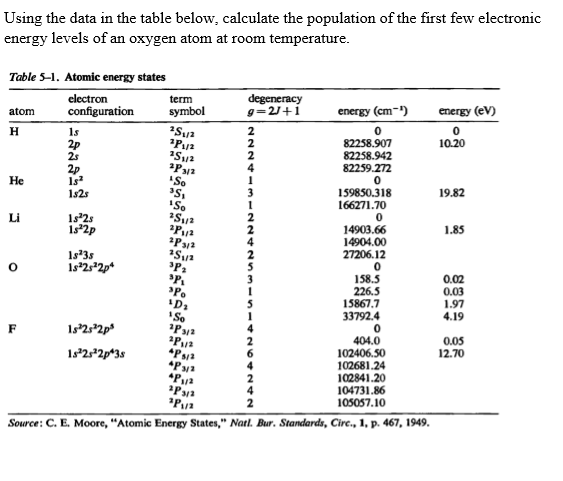


.PNG)