Amazon.com: ISO 13485 - Obstacle or Opportunity: How to live a QM system to benefit companies and employees eBook : Kalchschmid-Lehmann, Andreas: Kindle Store
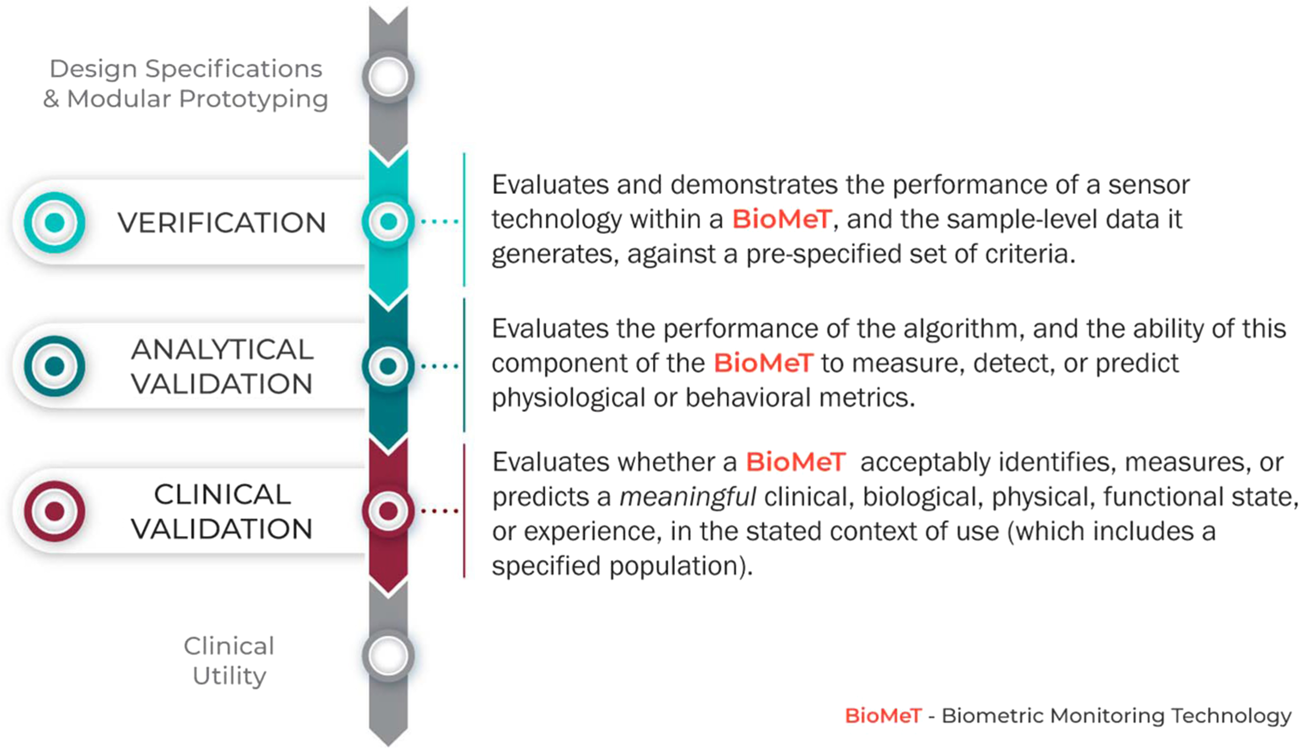
Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs) | npj Digital Medicine

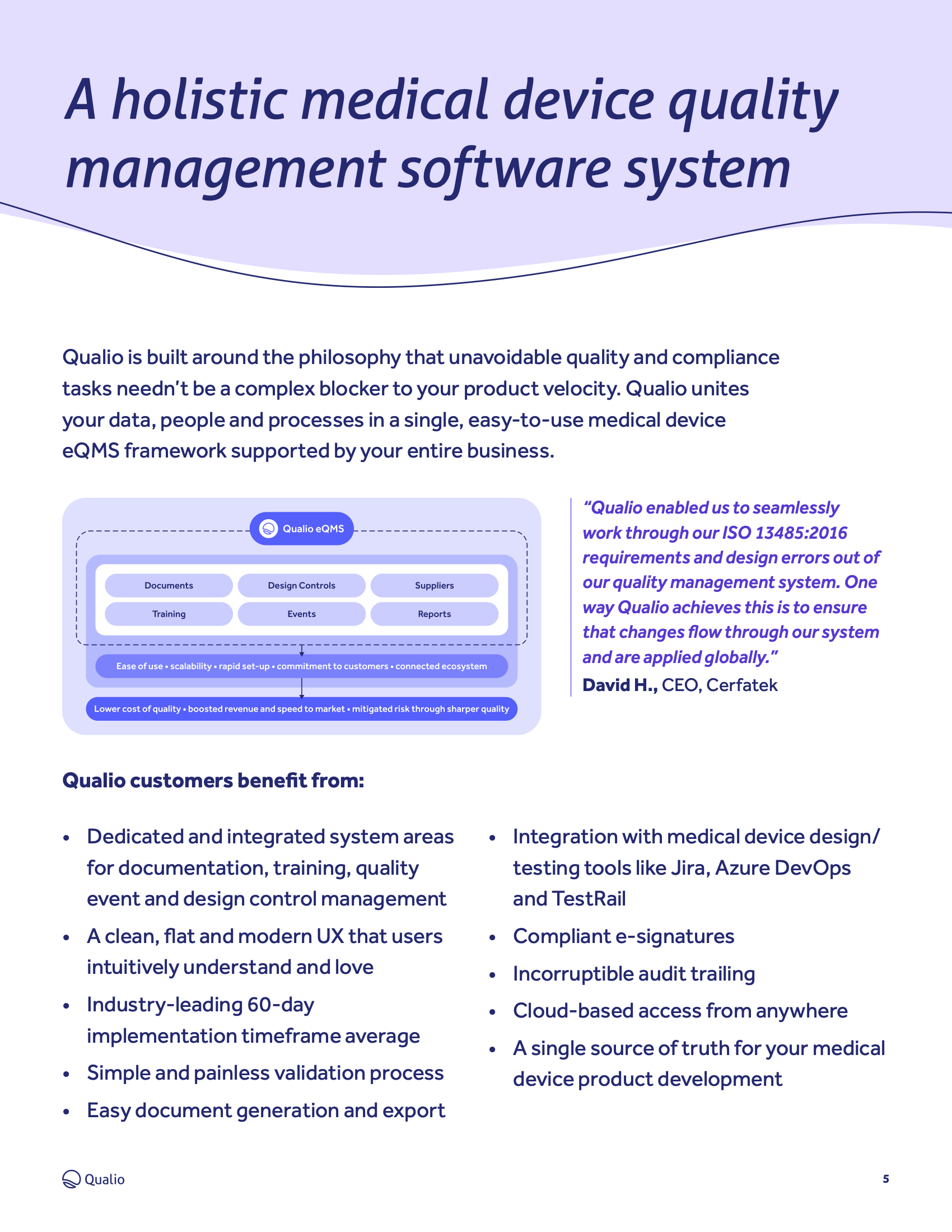
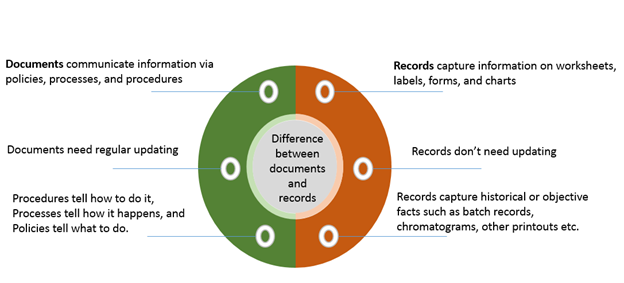
ISO 13485: Basics and How to Get Started (QMS for Medical Devices) | Process Street | Checklist, Workflow and SOP Software

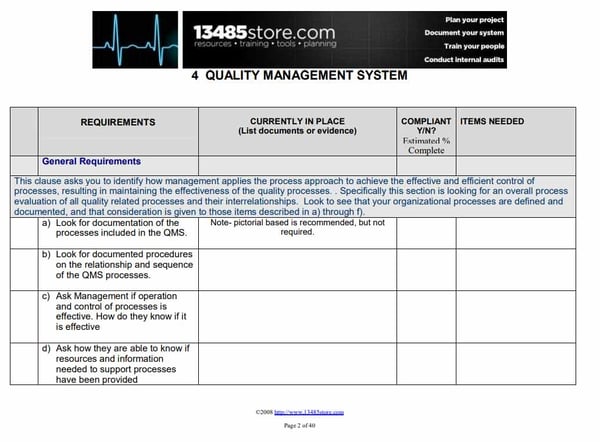
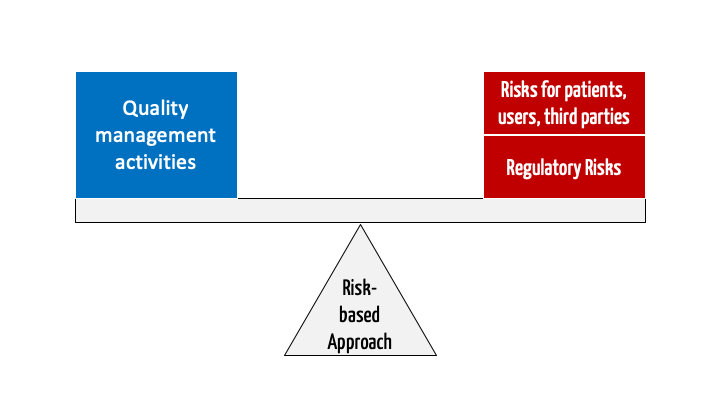
ISO 13485 – Medical Device Quality Management System Requirements – ISO Templates and Documents Download